Lewis Structure of the Peroxide Ion, O2(2) YouTube
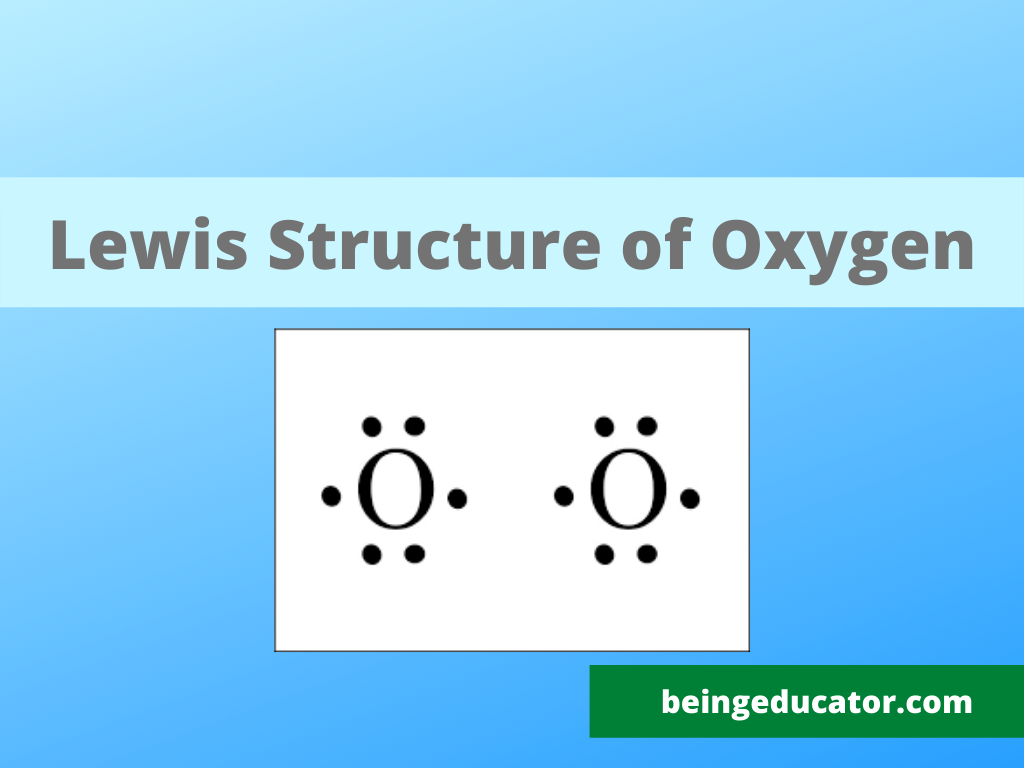
O2 Lewis Structure
Steps. Use these steps to correctly draw the O 2 Lewis structure: #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required #4 Convert lone pairs of the atoms, and minimize formal charges #5 Repeat step 4 if needed, until all charges are minimized, to get a stable Lewis structure
Single Oxygen Lewis Structure
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen Gas (Diatomic Oxygen).For the O2 structure use the periodic table to find the to.
o2 lewis structure Hospitallity Epic
Steps of drawing O2 lewis structure Step 1: Find the total valence electrons in O2 molecule. In order to find the total valence electrons in O2 (oxygen) molecule, first of all you should know the valence electrons present in a single oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily find the valence.
How to Draw O2 Lewis Structure? 1
2. ) Molecule Lewis Structure. Oxygen is a diatomic molecule and contains only two oxygen atoms. In the lewis structure of O 2 molecule, a double bond is located between oxygen atoms and each oxygen atom has two lone pairs in their valence shells. There are many things to learn when we draw the O 2 lewis structure.
:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)
Electron Dot Structure For Oxygen
Dalam molekul NH 3 terdapat sepasang elektron yang tidak digunakan (elektron bebas) sehingga disebut Pasangan Elektron Bebas (PEB). Tiga pasang elektron yang digunakan bersama oleh atom N dan atom H disebut Pasangan Elektron Ikatan (PEI). 2. Struktur Lewis Molekul H 2 O. Atom 8 O memiliki konfigurasi elektron 8 O:2, 6.

MENGGAMBAR STRUKTUR LEWIS O2BANK SOAL KE 10 YouTube
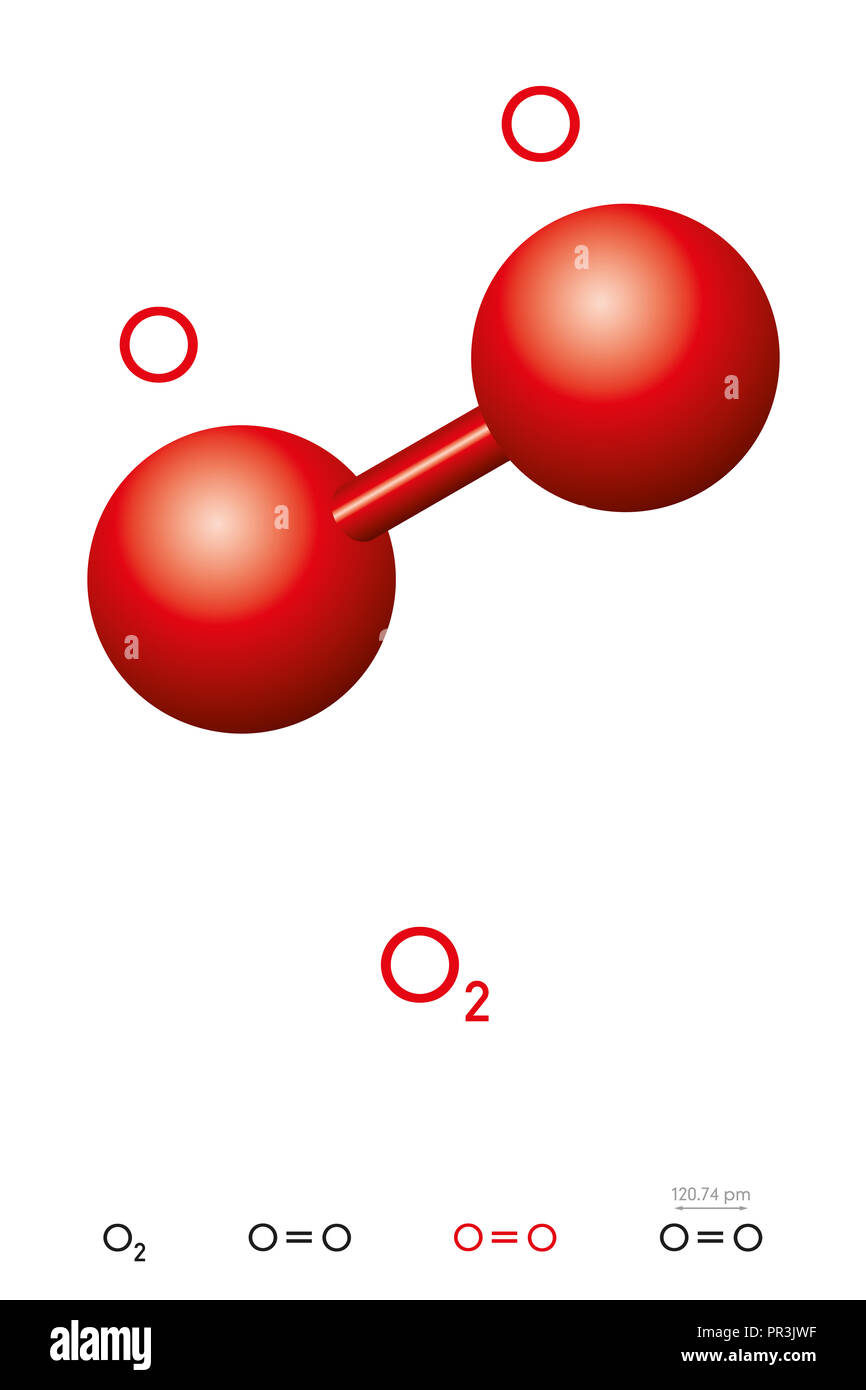
O2 is consist of two O atoms and the environment around both O atoms is the same. Both O atoms are sp3 hybridization. To satisfy the valency and complete the octet both O formed a double bond between them via sharing electrons. The shape of the molecule is linear and the bond distance between two O is 116 pm. ad.
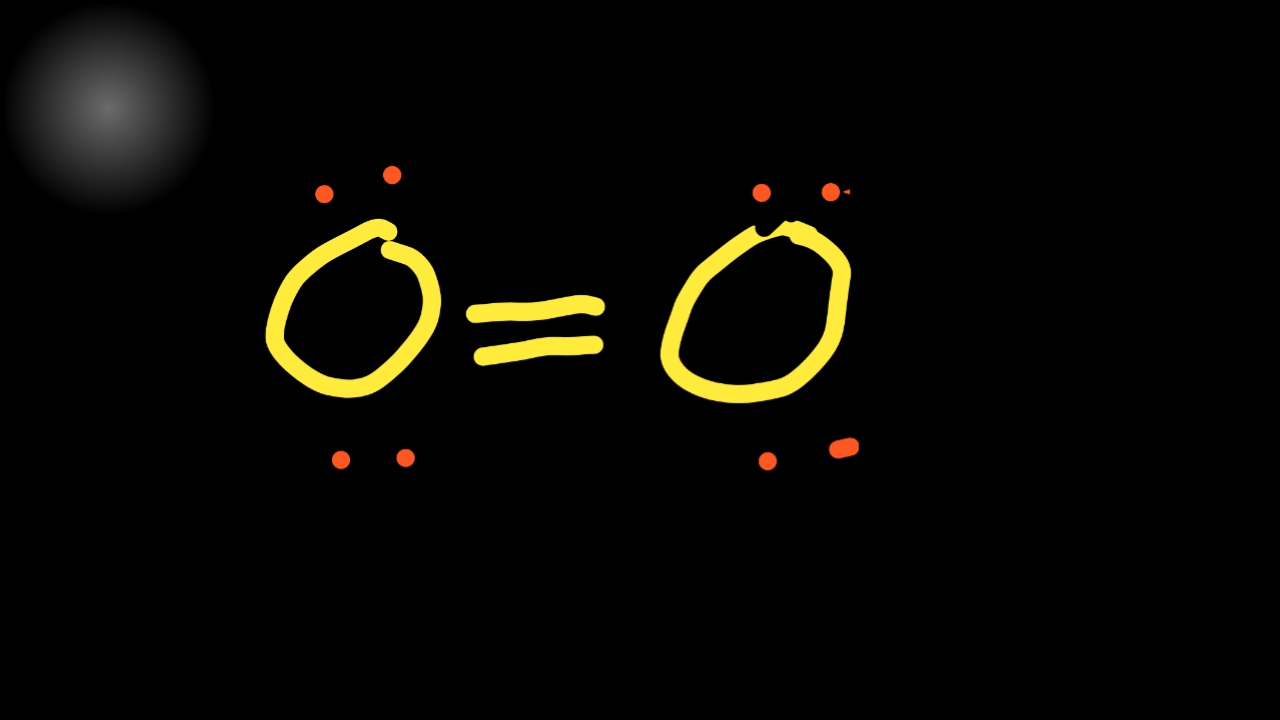
【2 Step】O2 Lewis StructureLewis Dot Structure for Oxygen(O,O2)Lewis Dot Structure of Oxygen
O2 Properties. The O 2 Lewis structure shows two oxygen atoms bonded in the same way to each other. It's perfectly symmetric. Generally, small symmetric molecules are nonpolar. The O 2 Lewis structure indicates that the O 2 molecule is perfectly symmetric. Therefore, O 2 is a nonpolar substance. Small nonpolar substances tend to be gasses.

Oxygen Atom Lewis Structure
Step #2: Select the center atom. While selecting the atom, you have to put the least electronegative atom at the center. But here in the O2 molecule, both the atoms are same. So you can consider any of the atoms as a center atom. So, let's assume that the oxygen which is on the right side is the central atom.

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Steps of drawing Lewis diagram. Find total valence electrons: It is two for each oxygen atom. Find how many electrons are needed: It is four for one O2 molecule. Look for the total number of bonds forming: Double covalent bonds are forming in an O2 molecule. Choose a central atom: Both the atoms will be central.

O2 (Oxygen) Lewis Dot Structure Science Trends
Lewis structure of a water molecule. Lewis structures - also called Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDs) - are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as.
Oxygen Atom Lewis Structure
1.2.1 Lewis Structure of Diatomic Molecules. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here: Figure 1.2a The Lewis structures of aluminum, tin, nitrogen, chlorine and bromine

Oxygen Valence Electrons (O) Oxygen Valency & Electron Configuration
A step-by-step explanation of how to draw the O2 Lewis Dot Structure (Oxygen gas).For the O2 structure use the periodic table to find the total number of val.

FileLewis O2.svg Wikimedia Commons
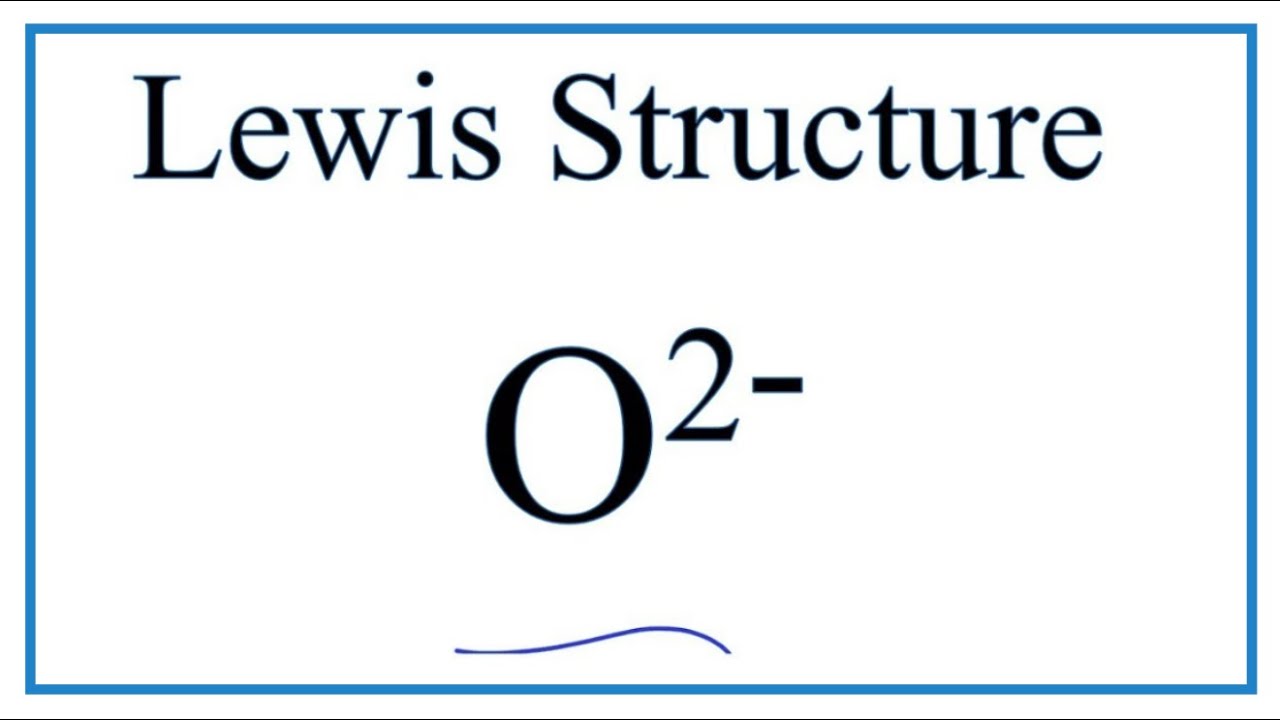
A step-by-step explanation of how to draw the O2- Lewis Dot Structure.For the O 2- structure use the periodic table to find the total number of valence elect.
O2 1 Lewis Structure Free Transparent PNG Clipart Images Download
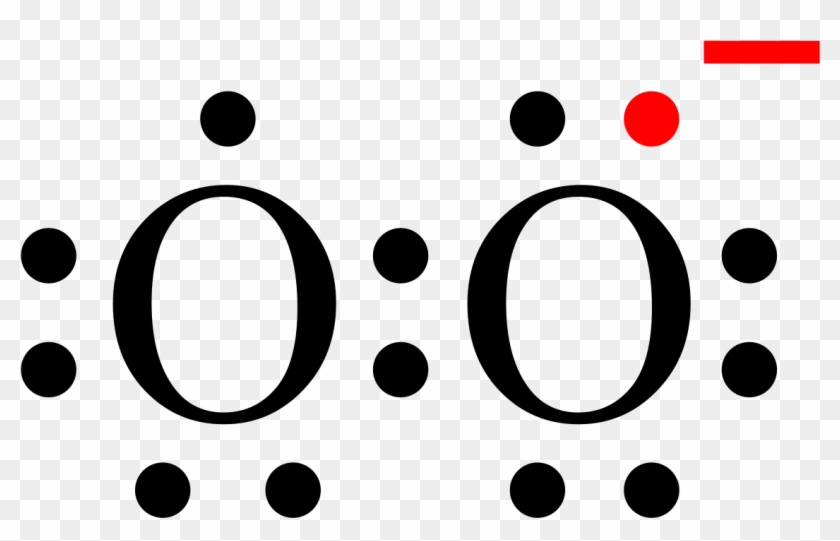
O. 2. 2-. (peroxide ion) Lewis Structure. O 22- (peroxide ion) anion contains only two oxygen atoms. Peroxide anion has -2 charge. In O 22- lewis structure, each oxygen atom has -1 charge and three lone pairs. Both oxygen atoms are joint through a single bond. In this tutorial, we are going to draw the lewis structure of O 22- ion step by step.

Oxygen Gas Oxygen Gas Lewis Structure
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.